When a drug leaves the lab and enters the market, its safety doesn’t end with approval. It must remain effective and safe for months-even years-under real-world conditions. That’s where stability testing comes in. This isn’t just paperwork. It’s a science-driven process that determines how long a medicine can sit on a shelf before it starts breaking down. And at the heart of it? Two non-negotiable factors: temperature and time.
Why Temperature and Time Matter in Stability Testing
Drugs aren’t static. They react to heat, moisture, and light. A pill stored in a hot bathroom cabinet might lose potency faster than one kept in a cool drawer. A liquid vaccine exposed to freezing temperatures could clump and become useless. Stability testing simulates these real-life scenarios to catch problems before patients get harmed. The goal is simple: find out how long a drug stays within its approved quality limits. If the active ingredient drops below 90% of its labeled amount, or if toxic breakdown products form, the product fails. That’s why regulators like the FDA and EMA require strict, repeatable conditions. No guessing. No shortcuts.ICH Q1A(R2): The Global Standard
Since 2003, the International Council for Harmonisation (ICH) has set the global benchmark with its Q1A(R2) guideline. It’s the rulebook used by the U.S., Europe, Japan, Canada, and more than 50 other countries. It doesn’t leave room for interpretation on temperature and time. For most solid oral drugs-like tablets and capsules-the long-term stability test runs at either:- 25°C ± 2°C with 60% RH ± 5% RH, or
- 30°C ± 2°C with 65% RH ± 5% RH
Accelerated Testing: The 6-Month Speed Test
Waiting two years for results isn’t practical when a new drug is ready to launch. That’s where accelerated testing comes in. It’s a forced stress test. The condition? 40°C ± 2°C and 75% RH ± 5% RH-for exactly six months. This isn’t meant to predict real-world aging. It’s designed to trigger degradation faster so scientists can spot potential issues early. Here’s the catch: if the drug shows a “significant change” during this six-month test, you must run an intermediate test. That means moving the samples to 30°C/65% RH for another six months. This step is critical. It helps determine if the drug is just sensitive to heat or if it’s fundamentally unstable.Refrigerated and Frozen Products: Different Rules
Not all drugs are stored at room temperature. Insulin, many biologics, and some vaccines need refrigeration. For these, the rules change. Long-term testing for refrigerated products happens at 5°C ± 3°C for at least 12 months. The accelerated condition? Not 40°C. That would destroy them. Instead, it’s 25°C ± 2°C with 60% RH for six months. This simulates what happens if a vaccine sits in a warm delivery truck for a day. Frozen products (below -15°C) have even more complex requirements. They’re tested for freeze-thaw cycles, ice crystal formation, and aggregation. These aren’t covered by ICH Q1A(R2)-they’re handled case by case, often requiring custom protocols.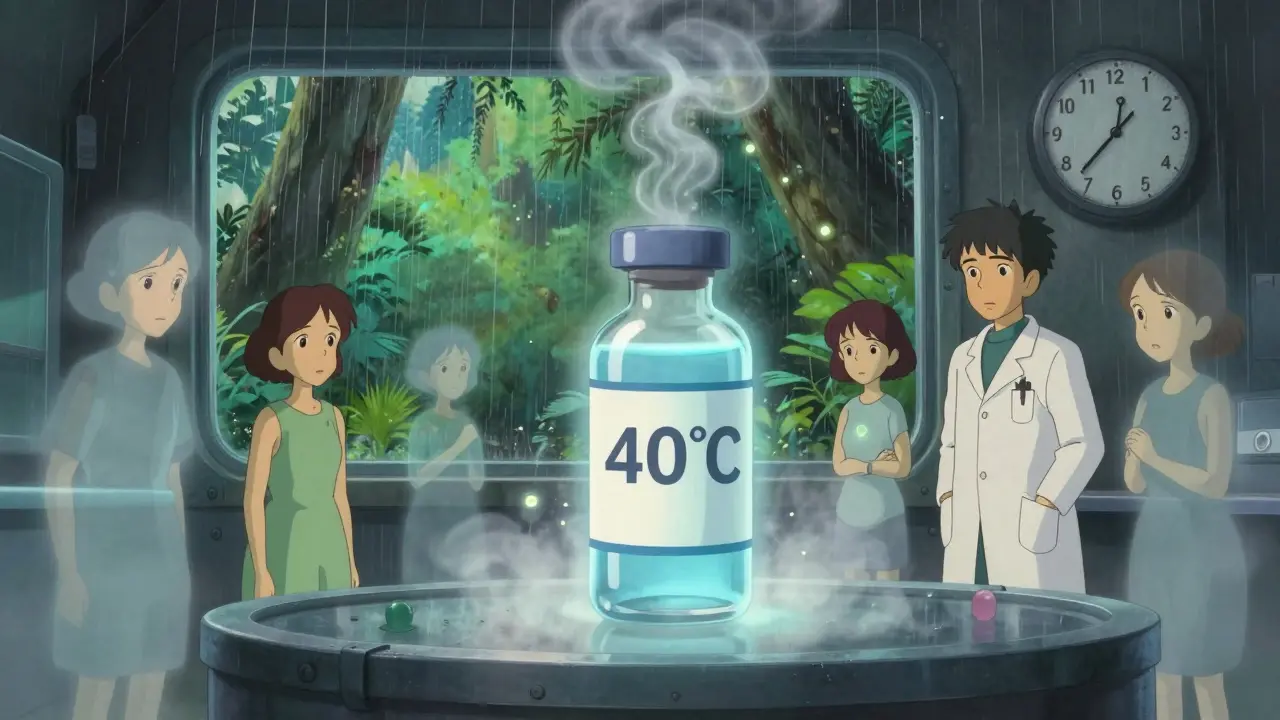
How Long Do You Have to Test?
The minimum testing schedule is 0, 3, 6, 9, 12, 18, 24, and 36 months. The first few time points (0, 3, 6) are frequent because that’s when most degradation happens. After 12 months, testing slows down unless there’s a red flag. At submission for approval, regulators require:- 12 months of long-term data (FDA requirement)
- 6 months of accelerated data
What Counts as a “Significant Change”?
This is where things get messy. ICH Q1A(R2) defines “significant change” as:- A 5% change in assay (potency)
- Any degradation product exceeding its qualification threshold
- Failure to meet physical appearance or dissolution specs
Real-World Failures and Why They Happen
In 2021, Teva had to recall 150,000 vials of Copaxone® because stability testing didn’t catch protein aggregation at 40°C. The drug was fine at room temperature-but in hot shipping containers, it degraded. The test conditions weren’t aggressive enough. Merck, on the other hand, used intermediate testing (30°C/65% RH) to catch a polymorphic shift in Keytruda®-a change in crystal structure that could’ve affected how the body absorbed the drug. That discovery saved millions in potential recalls. Temperature excursions are common. A 2023 survey of 142 stability labs found 78% had at least one chamber failure where the temperature drifted more than ±2°C. One degree off for a week can invalidate months of data. That’s why labs spend thousands on dual-loop humidity control and continuous monitoring systems.
Challenges with Modern Drugs
ICH Q1A(R2) was written for pills and capsules. It doesn’t fit modern therapies like mRNA vaccines, antibody-drug conjugates, or gene therapies. These are fragile. A single freeze-thaw cycle can destroy them. Standard 40°C tests are useless-they don’t mimic real-world stress. FDA warning letters to Amgen and Roche in 2021 and 2022 cited stability failures in biologics that weren’t predicted by traditional tests. Experts now argue the guidelines are outdated. The American Association of Pharmaceutical Scientists says 62% of solid dosage failures come from humidity cycling-not constant conditions. That’s something ICH Q1A(R2) doesn’t account for.What’s Next?
The ICH is working on Q1F, a proposed update expected in late 2024. It’s expected to address complex products and maybe even allow predictive modeling. Some companies are already using accelerated predictive stability (APS) studies-running tests at 50-80°C to forecast degradation in months, not years. The FDA is piloting real-time stability assessment using process analytical technology (PAT). If it works, companies might not need to wait 12 months to submit data. That could cut development time by 30-50%. But until then, the rules stay the same: 25°C or 30°C, 60% or 65% RH, 12 months minimum. No exceptions. No shortcuts. Because when a patient takes a pill, they trust it will work. Stability testing is how that trust is kept.Practical Tips for Getting Started
If you’re setting up a stability program:- Start with chamber qualification-IQ/OQ/PQ. Temperature must be within ±0.5°C across all shelves.
- Map your chambers. Hot spots and cold spots exist-even in new units.
- Use continuous monitoring with alarms. Don’t rely on daily logs.
- Choose your storage condition based on your target market. Don’t assume 25°C is enough.
- Plan for 12 months of long-term data before submission if targeting the U.S.
- Include intermediate testing if your accelerated test shows any change.
- Document everything. A stability dossier can be 500+ pages. Missing data means rejection.
Final Thoughts
Stability testing isn’t glamorous. It’s slow, expensive, and repetitive. But it’s the invisible guardrail between a safe medicine and a dangerous one. Temperature and time aren’t just numbers on a chart-they’re the difference between a patient getting relief and a patient getting sick. The rules haven’t changed in 20 years, but the drugs have. The next update to ICH Q1A(R2) might be the most important one yet.What are the standard temperature and humidity conditions for long-term stability testing?
The ICH Q1A(R2) standard allows two options: 25°C ± 2°C with 60% RH ± 5% RH, or 30°C ± 2°C with 65% RH ± 5% RH. The choice depends on the climatic zone of the target market. For example, tropical regions require the 30°C/65% RH condition, while temperate regions typically use 25°C/60% RH.
How long does accelerated stability testing last?
Accelerated stability testing runs for exactly six months at 40°C ± 2°C and 75% RH ± 5% RH. This is a forced degradation test designed to predict potential stability issues quickly. If significant changes occur, intermediate testing at 30°C/65% RH for six months is required.
Do refrigerated drugs follow the same stability conditions?
No. Refrigerated products are tested at 5°C ± 3°C for long-term stability. Their accelerated condition is 25°C ± 2°C with 60% RH for six months-not the 40°C used for room-temperature products. This prevents damage from unrealistic stress levels.
What is a “significant change” in stability testing?
A significant change is defined as: a 5% change in assay (potency), any degradation product exceeding its qualification threshold, or failure to meet physical or dissolution specifications. While the criteria are standardized, the interpretation of “qualification threshold” varies between labs and regulators, leading to inconsistencies.
Why do some companies test for 36 months if the minimum is 12?
Companies test beyond 12 months to establish a shelf life longer than one year. Regulatory agencies require data covering the entire claimed shelf life. If a product is labeled for 24 months, 24 months of data must be available. Many companies aim for 36 months to allow for flexibility in labeling and to meet global requirements.
Are ICH stability guidelines the same worldwide?
Yes, ICH Q1A(R2) is harmonized across major markets including the U.S. (FDA), Europe (EMA), Canada (Health Canada), and Japan (PMDA). However, submission requirements differ: the FDA requires 12 months of long-term data at submission, while the EMA allows 6 months under certain conditions, which can delay global approvals.
What happens if a stability study fails?
Failure can lead to regulatory actions including warning letters, product recalls, or withdrawal of marketing authorization. For example, in 2021, Teva recalled 150,000 vials of Copaxone® after stability testing revealed aggregation issues at elevated temperatures. Failed studies delay launches and cost millions in lost time and product.

So many labs skip the humidity mapping and just assume their chambers are fine. I’ve seen whole studies thrown out because one shelf was 5°C hotter than the rest. It’s wild how much we rely on equipment that’s barely monitored.
ICH Q1A(R2) is outdated. End of story. We’re testing mRNA vaccines with pill rules. This is why drugs fail in the field.
Of course the FDA wants 12 months of data. They’re terrified of liability. Meanwhile, the EMA’s just trying to get drugs to patients faster. One’s bureaucratic, the other’s pragmatic. Guess which one I’d trust with my life?
For anyone setting up a stability program: don’t just buy the cheapest chamber. Get the ones with dual-loop humidity control. I’ve seen companies save $20k upfront and lose $2M in recalls because they cut corners. It’s not an expense-it’s insurance.
The entire paradigm is fundamentally misaligned with the physicochemical behavior of biologics. ICH Q1A(R2) assumes first-order degradation kinetics under isothermal, isohumid conditions-but modern therapeutics exhibit polymorphic transitions, colloidal instability, and conformational denaturation that are non-linear, stochastic, and microenvironmentally sensitive. We’re applying 1990s engineering logic to 2020s molecular architectures. It’s not just inadequate-it’s epistemologically flawed.
They talk about ‘significant change’ like it’s some objective number, but I’ve seen labs reject a 4.9% potency drop and accept a 5.1% drop-same batch, same machine, different analyst. This isn’t science. It’s guesswork with a clipboard.
And don’t get me started on how they test frozen products. One freeze-thaw cycle? Try ten in real shipping. We’re not even trying to simulate reality anymore.
Someone needs to call this out. Not just in a white paper. In a courtroom.
Stability testing is the quiet backbone of modern medicine-and yet it’s treated like an afterthought. We spend billions on drug discovery, but when it comes to ensuring that pill doesn’t turn into poison on a shelf, we rely on 20-year-old templates, underfunded labs, and overworked technicians who check temperatures once a day.
It’s not just about compliance. It’s about dignity. The person taking that medicine doesn’t care about ICH guidelines. They care that it works. That’s the moral weight behind every data point. And if we’re not willing to treat this with the gravity it deserves, then we’re not healers-we’re just vendors.
Maybe the real question isn’t how to update the guidelines-but how to rebuild the culture around them.
I used to think stability testing was boring-until I saw a lab where the temp dropped to 1°C overnight and 300 vials of insulin went sideways. No alarms. No logs. Just a guy who noticed the fridge was cold the next morning. That’s the difference between a system and a gamble.
Here’s the thing: every time you see a drug recall, there’s a story behind it. Someone missed a humidity spike. Someone trusted a manual log. Someone didn’t map the hot spot. It’s not about being perfect-it’s about being paranoid. And if you’re not paranoid, you’re not paying attention.
Start small. Map one chamber. Set up a Slack alert. Buy a second thermometer. Don’t wait for a recall to wake you up. Your next patient might be your cousin.
Just wanted to say thanks for writing this. As someone who grew up in a country where meds are often stored in unrefrigerated shops under the sun, I never realized how much science goes into making sure a pill doesn’t turn toxic. This isn’t just regulation-it’s justice.