When you pick up a prescription, you might not realize there are different kinds of generic drugs on the shelf-and they don’t all cost the same. Two types dominate the market: authorized generics and first-to-file generics. They look identical, work the same way, but their pricing and market impact are wildly different. Understanding the difference can save you money-and help explain why your copay jumps or drops between refills.
What exactly is an authorized generic?
An authorized generic is the exact same drug as the brand-name version, made by the same company, in the same factory, with the same ingredients and packaging. The only difference? It’s sold without the brand name. Think of it like a store-brand soda made by Coca-Cola-same recipe, no logo. These drugs enter the market under the original brand’s New Drug Application (NDA), so they don’t need to go through the full generic approval process. That means they can launch quickly, sometimes even before the first traditional generic hits shelves.What’s a first-to-file generic?
A first-to-file generic is the first company to submit an Abbreviated New Drug Application (ANDA) to the FDA after a brand-name drug’s patent expires. Under the Hatch-Waxman Act of 1984, that company gets 180 days of exclusive rights to sell the generic version-no other generic can compete during that time. This exclusivity is a huge financial incentive. For big-selling drugs, those 180 days can mean hundreds of millions in revenue. But here’s the catch: during that window, the first-filer often charges prices that are still relatively high. Why? Because they’re the only game in town.Price differences: the numbers don’t lie
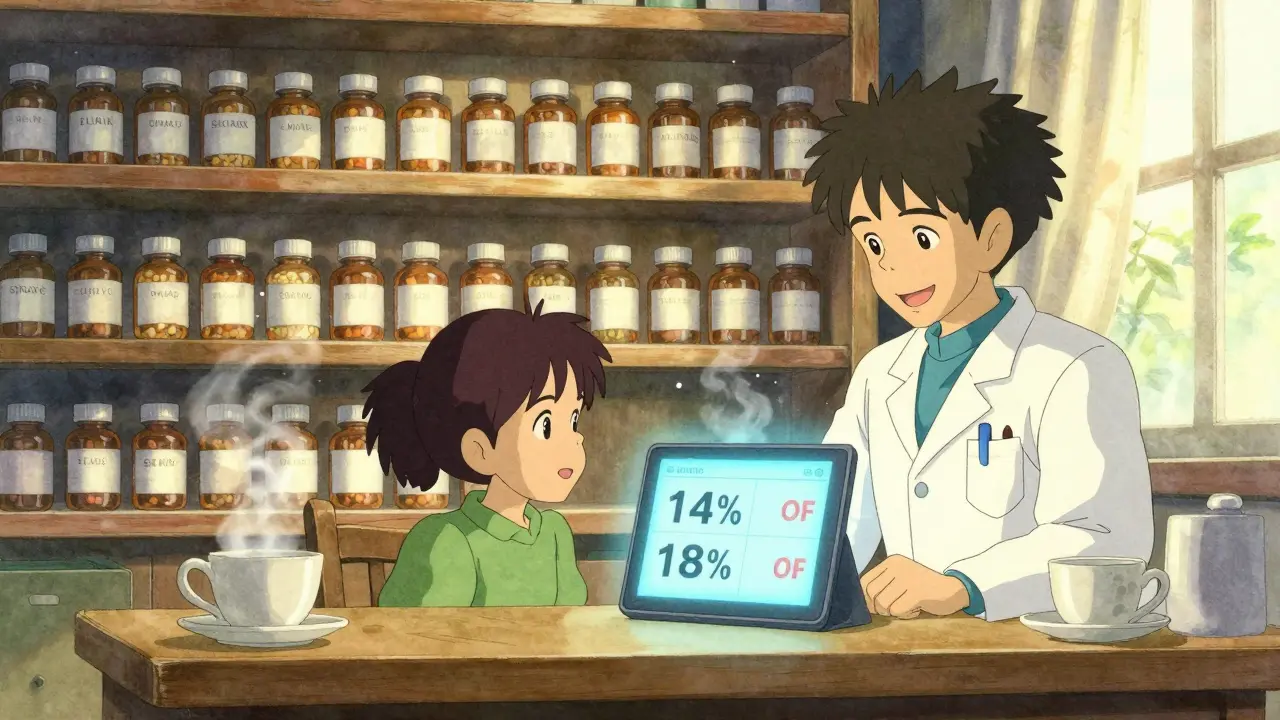
The Federal Trade Commission (FTC) tracked over 95 drugs between 2005 and 2013 to see how prices changed when both types of generics were on the market. The results were clear. When only the first-to-file generic is selling, the average retail price is about 14% lower than the brand-name drug. But when an authorized generic enters the market during that same 180-day window? The price drops to 18% below brand. That’s a 4 percentage point jump in savings-right in your pocket. The savings get even bigger when you look at what pharmacies actually pay for the drugs. Without authorized generic competition, pharmacies pay about 20% less than brand prices. With an authorized generic in the mix? That jumps to 27% less. That’s not just a small discount-it’s a major shift in pricing power. And it doesn’t stop there. The FTC found that when both generics are selling, retail prices are 4-8% lower than when only the first-filer is around. Wholesale prices? They drop 7-14%. For a $100 brand-name drug, that could mean paying $82 instead of $86. Multiply that across millions of prescriptions, and you’re talking about billions saved in the healthcare system.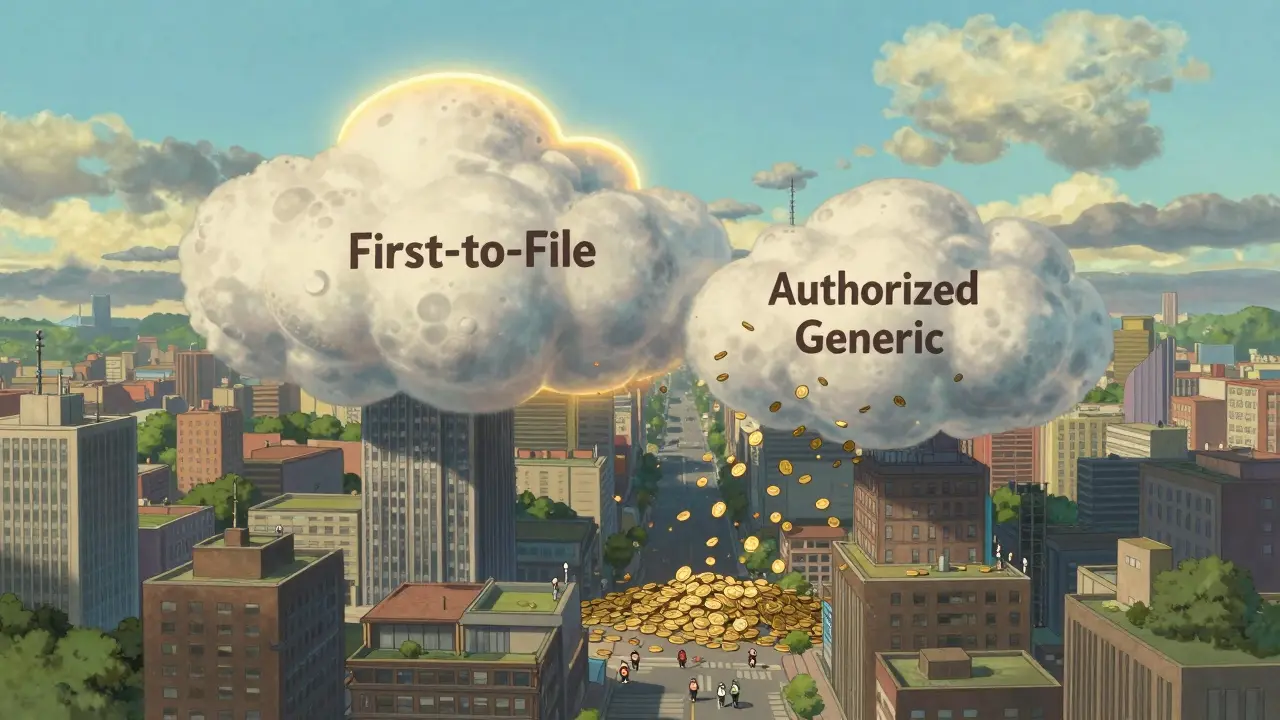
Why does this matter for pharmacies and patients?
Pharmacies don’t just benefit from lower prices-they make more money too. When the first-to-file generic enters, pharmacy profit per prescription goes up. When an authorized generic joins the market, profits climb even higher. Why? Because competition forces both generics to lower their prices, but the cost to the pharmacy stays low. That means pharmacies can sell more, make more margin, and still pass savings to customers. For patients, it’s simple: more competition = lower out-of-pocket costs. If your insurance uses a tiered system, moving from a brand to a generic can drop your copay from $50 to $10. Add an authorized generic into the mix, and you might see it drop to $5-or even free, depending on your plan.What about long-term effects?
You might think the brand-name company would never let this happen-why help undercut their own product? But here’s the twist: many brand manufacturers launch authorized generics as part of legal settlements with generic companies. Instead of fighting patent lawsuits for years, they agree to let their own generic version enter the market early. This cuts legal costs and keeps revenue flowing, even if it’s from a generic label. The FTC studied this closely and found something surprising: even though authorized generics cut into the first-filer’s profits by 40-52% during the exclusivity period, they didn’t reduce the number of patent challenges by generic companies. In other words, the financial incentive to be first-to-file is still strong enough to drive competition. That’s good news for patients-it means the system still works. Some critics worry that authorized generics might delay other generics from entering the market. But data from the FDA and Health Affairs show that when more competitors join-four, six, or more-the price drops to 95% below brand levels. Authorized generics don’t block that; they speed it up.What about drug quality?
No difference. Authorized generics are identical to the brand-name drug in strength, safety, and effectiveness. They’re made in the same facility, with the same quality controls. The FDA requires them to meet the same standards as any other generic. There’s no hidden compromise. First-to-file generics are just as safe. They’re bioequivalent, meaning they deliver the same amount of medicine into your bloodstream at the same rate. The only real difference is timing and price.
How does this affect your next prescription?
If your doctor prescribes a brand-name drug, ask if a generic is available. If you’re told there’s a generic, ask: Is it the first-to-file version, or is there an authorized generic too? Pharmacists can tell you. If both are available, the authorized generic is often cheaper. Also, check your insurance formulary. Some plans favor authorized generics because they’re cheaper. If your plan lists both, you might pay less with the authorized version-even if it’s technically the same drug. And if you’re on a high-dose or long-term medication? Even a 5% price difference adds up. A $150 monthly drug that drops to $140 saves you $120 a year. With an authorized generic, you might save $180 or more.What’s changing in 2025?
The FDA’s Generic Drug User Fee Amendments (GDUFA) have sped up approvals. In 2012, only 20% of generic applications got approved on the first try. By 2025, that number is up to 66%. That means more generics enter the market faster-and more competition follows sooner. That’s good news. The more generics there are, the lower prices go. Authorized generics are part of that chain. They don’t slow things down; they jump-start them. The FTC is still watching. In 2022, Commissioner Alvaro Bedoya said the agency remains alert to any anti-competitive behavior. So far, authorized generics have passed the test: they’ve lowered prices without harming innovation or access.Bottom line: which one saves you more?
If you’re choosing between a first-to-file generic and an authorized generic, go with the authorized one-when it’s available. It’s the same drug, often cheaper, and backed by the same manufacturer as the brand. You’re not sacrificing quality. You’re getting more value. And if you’re wondering why your copay changed last month? It’s probably because an authorized generic just entered the market. That’s not a glitch-it’s the system working the way it should.Are authorized generics the same as brand-name drugs?
Yes. Authorized generics are made by the brand-name manufacturer using the exact same formula, ingredients, and production process. The only difference is the label-they’re sold without the brand name. The FDA requires them to be identical in safety, strength, and effectiveness.
Why are authorized generics sometimes cheaper than first-to-file generics?
Because they introduce competition during the first-to-file’s 180-day exclusivity period. Without competition, the first-filer can charge higher prices. When an authorized generic enters, both companies lower prices to win market share. That drives down costs for patients and pharmacies.
Can I ask my pharmacist for an authorized generic?
Absolutely. Pharmacists track which generics are available and their pricing. If an authorized generic exists for your prescription, ask if it’s an option. Many insurance plans prefer them because they’re cheaper, which can mean lower copays for you.
Do authorized generics delay other generics from entering the market?
No. Studies by the FTC show that authorized generics don’t reduce the number of patent challenges or slow down other generic entries. In fact, they often encourage more competition. Once the 180-day exclusivity ends, multiple generics flood the market-and prices drop even further.
Are authorized generics covered by insurance?
Yes. Authorized generics are treated like any other generic drug by insurance plans. They’re usually on the lowest tier of formularies, meaning lower copays. Some plans even list them separately because they’re cost-effective.

Yo I just got my insulin prescription and switched to the authorized generic last month-copay dropped from $45 to $7. Like, WTF? I didn’t even ask for it, the pharmacist just handed it over. Now I’m telling everyone I know. This system actually works for once.
Bro this is why I love capitalism when it’s not broken 🤝
Same drug, same factory, same pills… but suddenly it’s cheaper? The brand just slapped a new label on it and called it a day. No magic. No science. Just competition. We need more of this. 🙌
While the empirical data presented by the FTC is certainly compelling, one must consider the broader macroeconomic implications of authorized generics on patent incentive structures. The erosion of monopolistic pricing windows may inadvertently disincentivize innovation in pharmaceutical R&D, particularly for high-risk, high-reward therapeutic domains.
Okay but let’s be real-why does this even exist? The brand companies are just playing games. They make the generic themselves so they don’t lose money, then act like they’re doing us a favor. Meanwhile, I’m still paying $10 for a pill that costs 12 cents to produce. This isn’t healthcare. It’s corporate theater.
So if I ask my pharmacist for the authorized generic, they’ll know which one it is? I always just grab the cheapest one without thinking.
THEY’RE LYING TO US. 😳
Authorized generics are a trap. Big Pharma lets ONE of their own sell cheap so you stop complaining… then they raise prices on everything else. I’ve seen it. It’s a scam. 🕵️♀️
Let me get this straight-we’re celebrating a drug company selling its own product under a different label as some kind of victory? The Hatch-Waxman Act was supposed to break monopolies, not let pharma play rock-paper-scissors with its own patents. Congrats, we’ve turned healthcare into a board game.
I’ve never heard of authorized generics before. This makes me want to learn more about how my prescriptions work. Thanks for explaining it so clearly.
THIS IS WHY WE WIN 💪
My grandma switched to the authorized generic for her blood pressure med and now she’s saving $120 a year. She bought herself a new pair of shoes with it. That’s real impact. More of this, please!
Why do we even need to know this the brand name is always better anyway
In Nigeria we just buy from the street and pray but you guys overcomplicate everything with labels and forms and FDA and stuff
Wait so… if the authorized generic is made by the same company… then isn’t it just… the brand… but cheaper?? Like… why do they even bother making the brand then?? I’m so confused now… 😵💫